Apple's "It's Glowtime"event may have spotlighted the iPhone 16, but the most groundbreaking announcement came from the AirPods Pro 2.
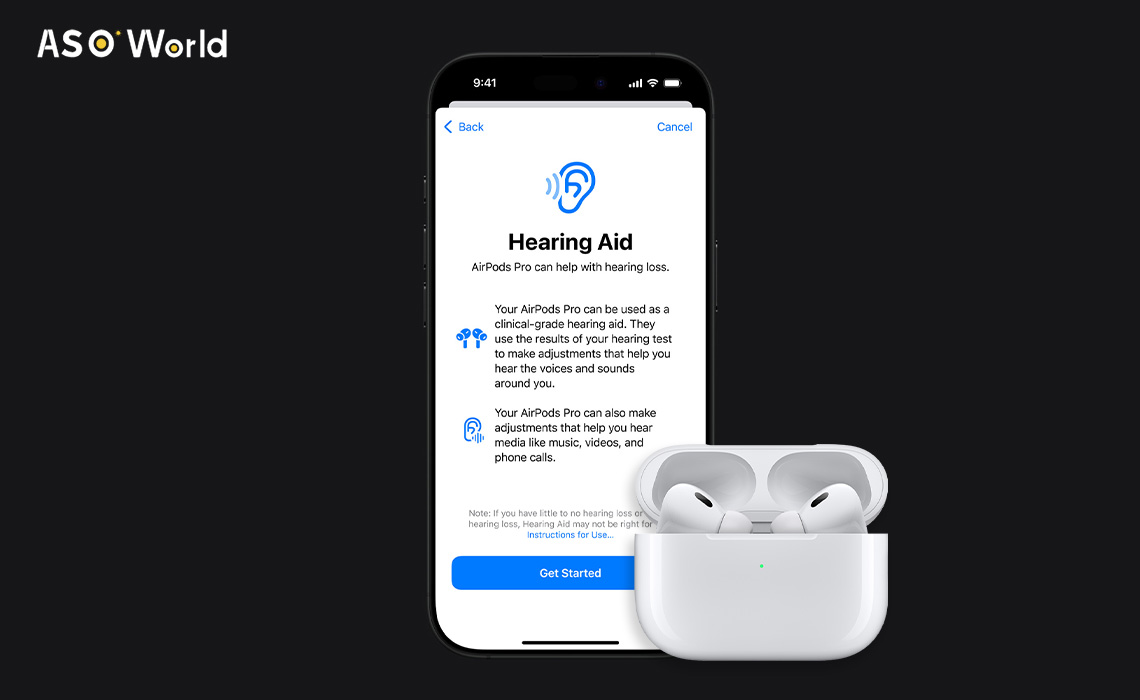
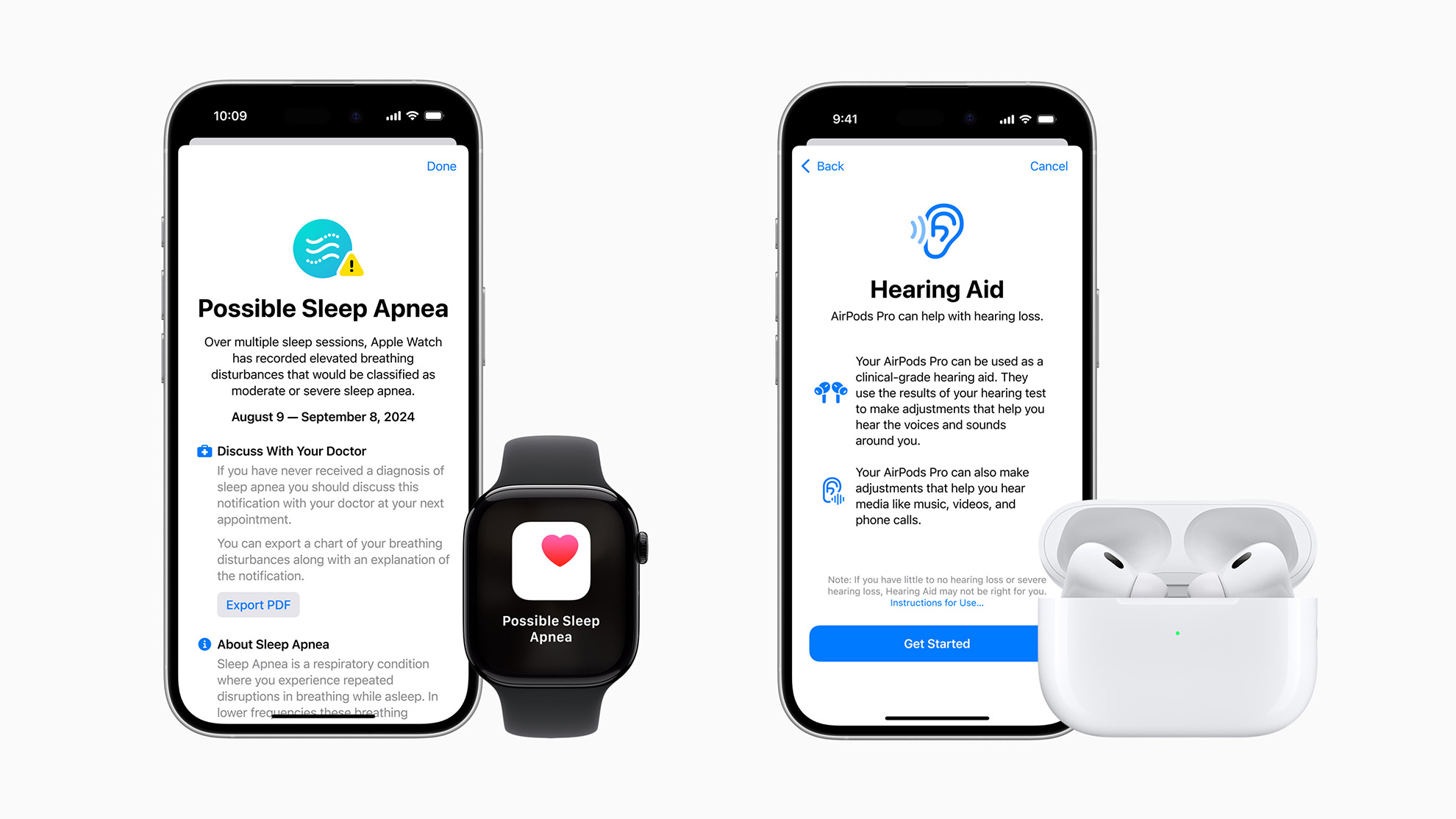
The U.S. Food and Drug Administration (FDA) has granted approval for these earbuds to function as over-the-counter hearing aids, thanks to a new software update.
FDA Approval and Public Health Impact
FDA's Groundbreaking Decision
The FDA announced its approval of Apple's software update, labeling it as the "first over-the-counter (OTC) hearing aid software device."
This decision follows the FDA's October 2022 ruling, which allowed the sale of hearing aids without a prescription. This move has paved the way for more accessible hearing solutions.
Public Health Significance
"Hearing loss is a significant public health issue impacting millions of Americans," said Michelle Tarver of the FDA.
The new software aims to make hearing support more accessible and acceptable for adults with perceived mild to moderate hearing loss.
Features and Functionality
Hearing Test and Personalized Profiles
The new feature will be accessible through iOS 18 settings when a pair of AirPods Pro 2 are connected. Users can take a hearing test via the earbuds, or upload test results from a doctor, to receive a personalized audio profile. This profile adjusts different frequency levels to amplify key sounds, such as the human voice.
Hearing Protection Mode
In addition to its hearing aid functionality, the AirPods Pro 2 will also include a Hearing Protection mode. This feature will safeguard users' ears in loud environments, such as concerts, while preserving the natural and vibrant sound of live performances.
Clinical Validation and Future Rollout
Clinical Study Results
The FDA's approval was based on a clinical study involving 118 subjects with perceived mild to moderate hearing loss.
The study found that users who employed Apple's self-fitting strategy for the hearing aid feature experienced similar benefits to those who received professional fitting.
Upcoming Features and Availability
Apple plans to roll out the hearing aid feature for the AirPods Pro 2 this fall. The update will also include other enhancements aimed at improving hearing health, reinforcing Apple's commitment to this crucial aspect of public health.
Editor's Comments
Apple's initiative to incorporate hearing aid functionality into the AirPods Pro 2 is a significant step toward reducing the stigma and cost associated with traditional hearing aids.
With approximately 1.5 billion people globally experiencing some level of hearing loss, this development could have a profound impact on public health.
The FDA's approval under De Novo classification highlights the novelty and low-to-moderate risk associated with this innovative solution.
As Apple continues to integrate health-focused features into its products, it sets a new standard for consumer electronics that serve both entertainment and health needs.